This website is intended for healthcare professionals. If you are a member of the public, please visit our public website.
Feraccru clinical trial data
In the randomised phase-3 AEGIS IBD clinical trial programme, the efficacy and tolerability of Feraccru was assessed in the treatment of iron deficiency anaemia in patients with inflammatory bowel disease who had previously failed to respond, or had been intolerant to previous oral ferrous products (OFP) therapy1
Feraccru was shown to be effective and well-tolerated at both 12 and 64 weeks1,2
Mean Hb concentration from baseline to Week 12 (mean ± SD)1
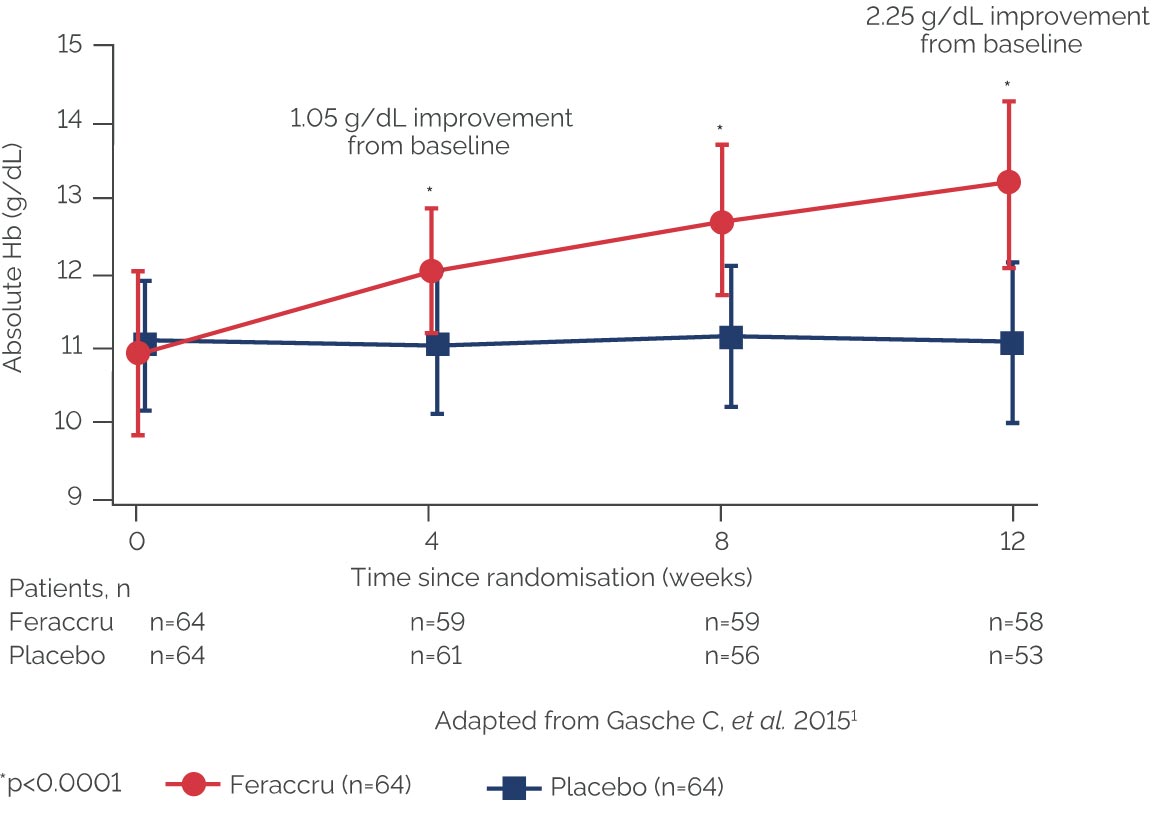
The ABPI states that particular care should be taken with graphs and tables to ensure that they do not mislead, for example by their incompleteness or by the use of suppressed zeros. A suppressed zero on the Y axis has been used in this instance because Feraccru is only indicated in patients with a haemoglobin level ≥9.5g/dL. This is why the graph starts at 9g/dL.
The median time to Hb normalisation was <2 months with Feraccru1
At Week 12:
- 66% of the Feraccru group had normal Hb levels1
- Hb concentrations increased by ≥1 g/dL in 78% of patients in the Feraccru group1
- Efficacy of Feraccru was not affected by IBD type, severity or time since flare1
Mean Hb concentration over 64 weeks’ treatment2
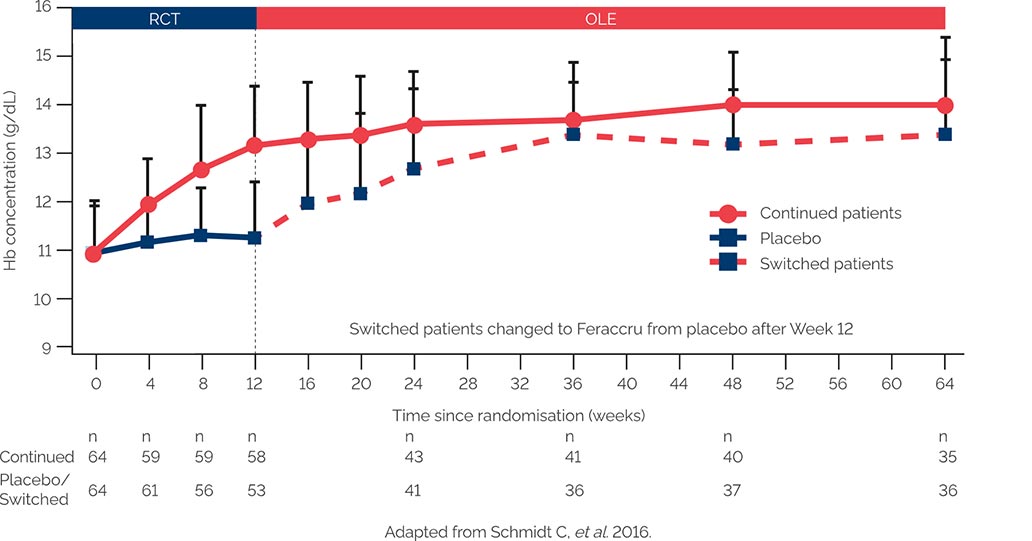
The primary efficacy endpoint was absolute change in Hb concentration from baseline to Week 64 (12 plus 52 weeks)2 The ABPI states that particular care should be taken with graphs and tables to ensure that they do not mislead, for example by their incompleteness or by the use of suppressed zeros. A suppressed zero on the Y axis has been used in this instance because Feraccru is only indicated in patients with a haemoglobin level ≥9.5g/dL. This is why the graph starts at 9g/dL.
97% of patients were compliant with long-term treatment in both groups2
Incidence of AEs at Week 12 (pre-planned safety set)1
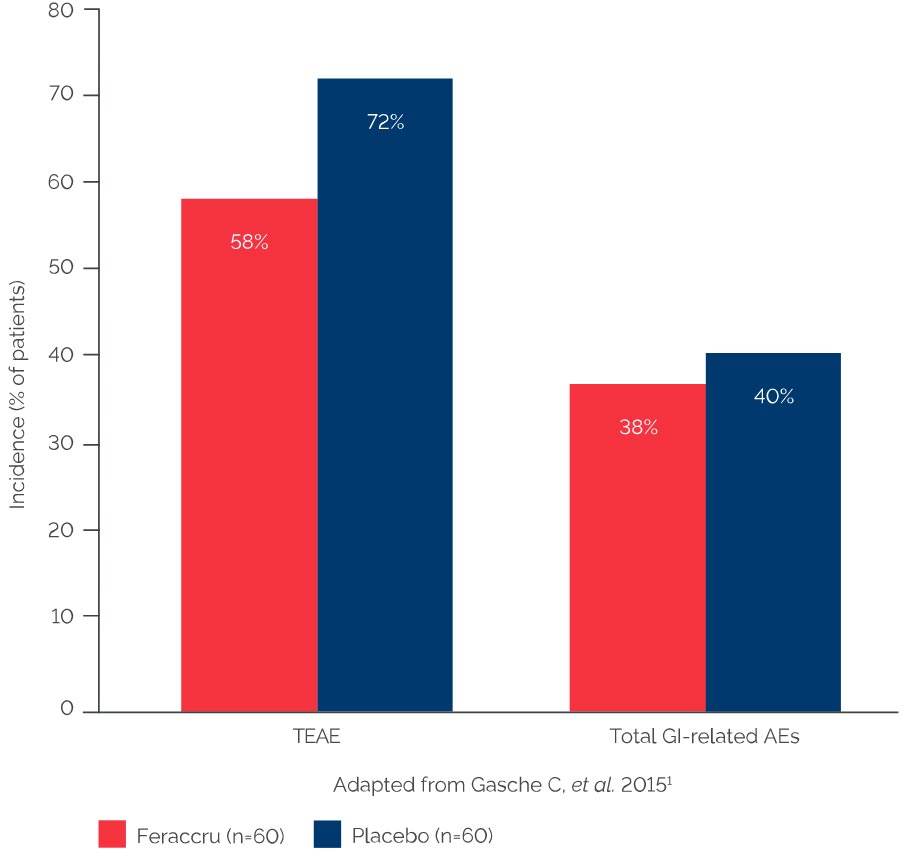
No negative impact on disease-specific quality of life scores or disease severity was observed for the Feraccru study arm1
More than 75% of patients completed 64 weeks of treatment2
AEGIS IBD 1 & 21
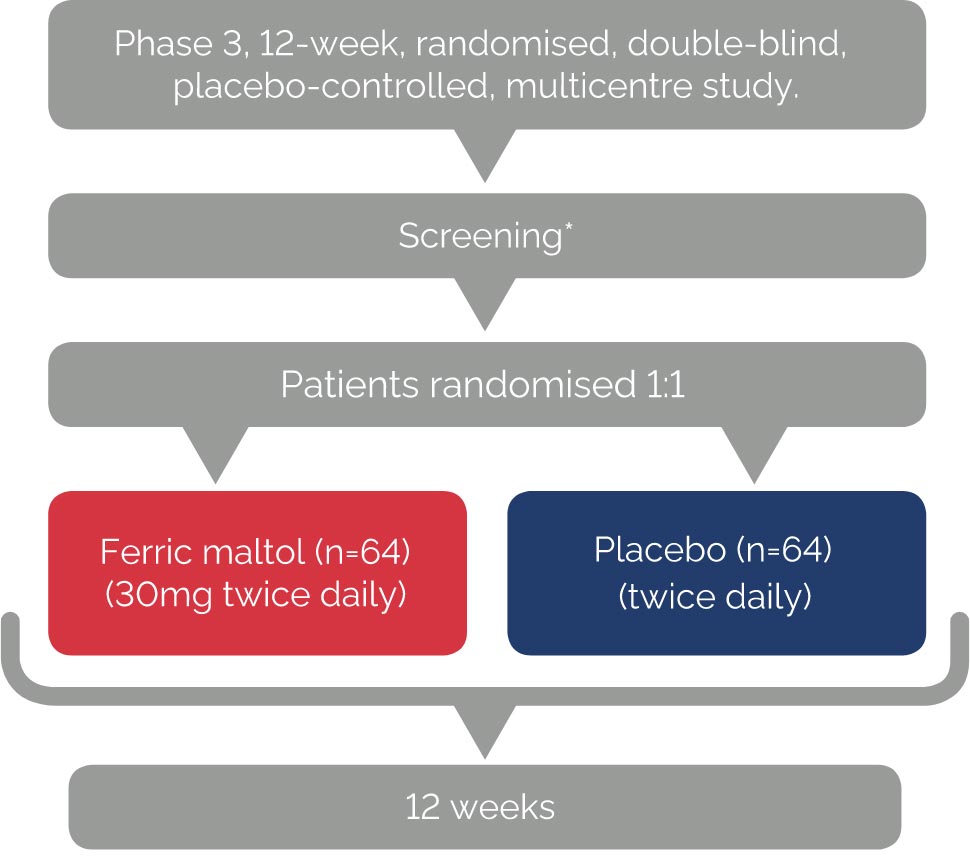
Primary endpoint
• Change in Hb concentration from baseline to Week 12
Secondary endpoints
• Changes in Hb concentration from baseline to Weeks 4 and 8
• Serum ferritin concentration
• Percentage transferrin saturation (TSAT)
*7-14 day screening period.
AEGIS IBD long-term extension2
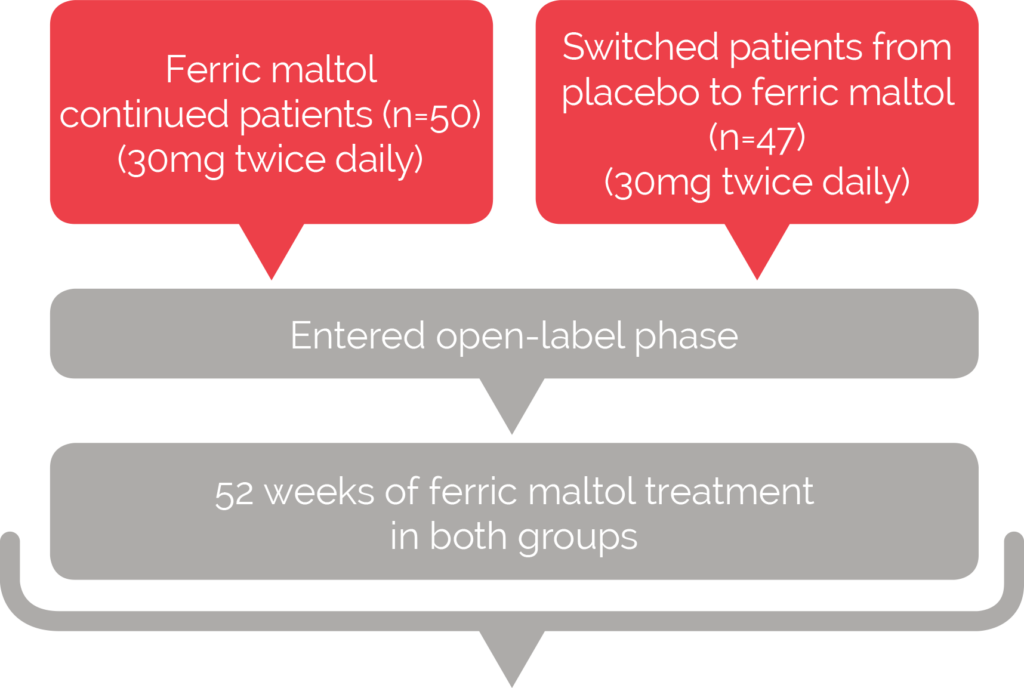
Main efficacy endpoint
• Absolute change in Hb from baseline to Week 64
(12 plus 52 weeks)
Secondary endpoints
• Proportion of patients achieving normal haemoglobin levels
• Absolute serum ferritin concentration and transferrin
saturation at 4- and 12-weekly intervals
• Disease specific quality of life at randomisation,
Week 12 and 12-weekly intervals thereafter