This website is intended for healthcare professionals. If you are a member of the public, please visit our public website.
When to use Feraccru
How to take Feraccru
Feraccru should be taken twice a day and swallowed whole on an empty stomach (with half a glass of water). In practice, this would mean taking Feraccru one hour before eating, or two hours after eating. Treatment should be continued for as long as necessary to replenish the body iron stores according to blood tests1
Oral iron should be continued for at least 3 months after IDA has been corrected so that stores are adequately replenished2
Feraccru should not be used in patients with inflammatory bowel disease (IBD) flare or in IBD patients with haemoglobin (Hb) <9.5 g/dL.1
Iron preparations in excess may cause toxicity especially among children. Feraccru must not be administrated to children.1
Special care should be taken if other dietary and/or iron salt supplementation are used concurrently.1
Feraccru has not been studied in patients with impaired renal and/or hepatic function.1
This medicinal product contains lactose: patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicine.1
This medicinal product also contains Allura Red AC (E129) and Sunset Yellow FCF (E110): these may cause allergic reactions.1
Iron Deficiency Anaemia in Inflammatory Bowel Disease – suggested algorithm
Please refer to summary of product characteristics before prescribing
Feraccru is indicated for the treatment of ID in adults, so can be used for the treatment of IDA in adult patients with IBD. Feraccru can also be used second line as an alternative oral option in patients where there has been a lack of efficacy, or there is intolerance to oral ferrous salts. In these patients, it should be considered as an alternative to IV iron if their Hb levels are ≥9.5 g/dl.1
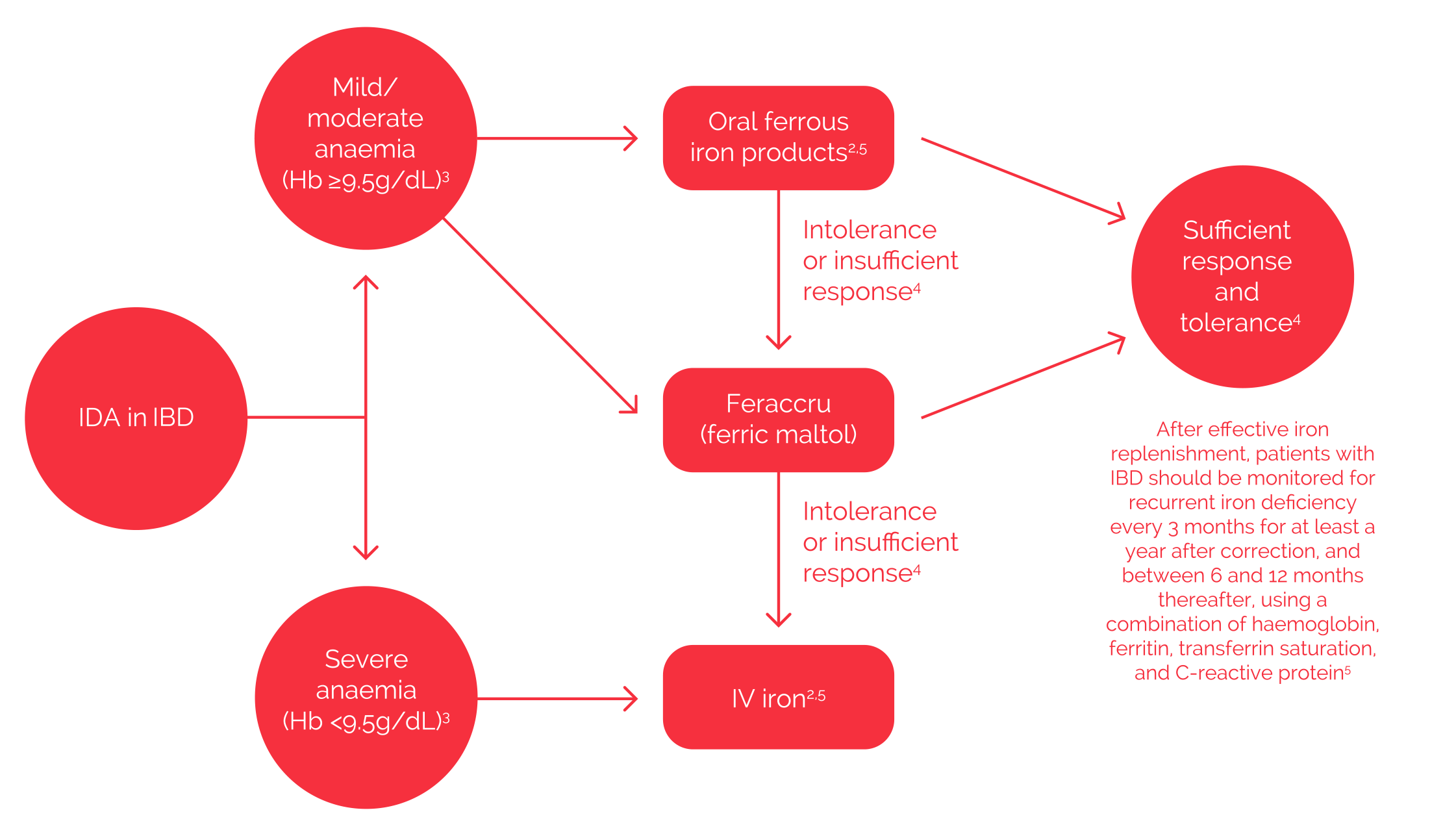
Diagram does not represent clinical guidelines for the management of IDA in IBD.
Guidelines to improve the management of IDA